Abstract
Introduction: Ibr and ven are 1 st generation BTK and BCL2 inhibitors respectively that are approved for use in patients with CLL. They are both oral agents that have been shown to work synergistically together with no unexpected toxicities beyond what is already known about each of them. Their combination is very effective in the frontline treatment of CLL including fixed duration therapy for 15 cycles which produces high complete remissions (CR) and undetectable minimal residual disease (uMRD) rates as well as high progression free survival (PFS) and overall survival (OS) independent of high-risk features [Allen JH, et al. EHA virtual annual meeting 2021; Jain N, et al N Engl J Med 2019]. There is also data to support the use of this combination in the rel/ref setting [Hillmen P, et al. J Clin Oncol 2019] and here we present results of a phase 2 trial of fixed duration therapy with ibr+ven in rel/ref CLL (NCT03045328).
Methods: Patients with rel/ref CLL with indications for treatment were enrolled at Stanford Cancer Institute and City of Hope National Medical Center. Treatment was initiated with ibr monotherapy (420mg daily) for 8 weeks after which ven ramp up was introduced. Ven was escalated to full dose (400mg daily) over 5 weeks and the combination of ibr+ven was then continued for a total of 2 years. Treatment was stopped in all patients at week 118 and all patients were evaluated 30 days later to complete study follow up. The primary endpoint was the assessment of CR rate at week 62. Secondary endpoints included overall response rate (ORR), duration of response (DOR), PFS, OS, evaluation of adverse events (AEs), as well as rate of uMRD in the bone marrow at week 62 and week 117 of treatment by flow cytometry (10 -4 sensitivity [uMRD4]) and Clonoseq sequencing (10 -6 sensitivity [uMRD6]).
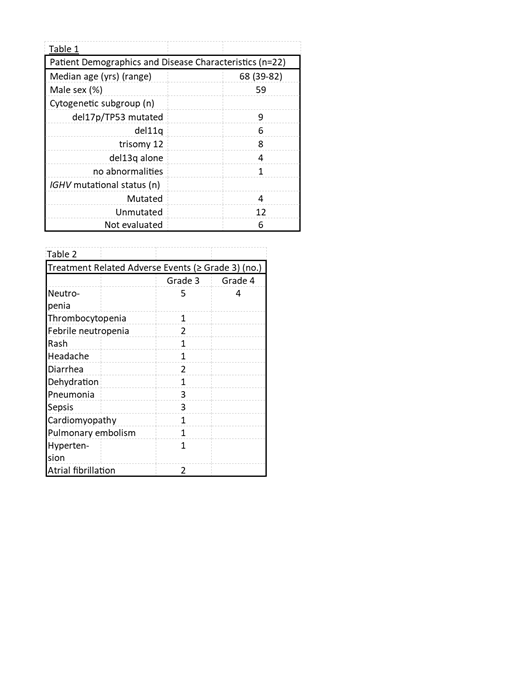
Results: A total of 22 patients were enrolled. Median age of the patients was 68 (range 39-82 years). Median prior treatment regimens were 1 (range 1-3). Eleven patients received prior FCR as 1 st line therapy, 9 received BR, 1 received FR, and 1 had received rituximab alone. None had prior BTK inhibitor or ven. Baseline characteristics are shown in Table 1.
Twenty-one patients initiated ven. One patient withdrew consent prior to the start of ven. Eighteen patients completed full trial treatment. Three patients discontinued treatment for these reasons: Hodgkin's transformation, kidney failure, need for a coronary stent. Five patients interrupted dosing due to toxicity and five patients had dose reductions. Three patients reduced ibrutinib, 2 due to rash and 1 due to recurrent atrial fibrillation, and 5 patients reduced ven due to fatigue, neutropenia (2), or diarrhea (2).
CR rate (intention to treat [n=22]) at week 62 was 55% while the ORR was 91%. PFS and OS were 95% and 100% at 2 years. After 1 year of combination therapy, 13 of 20 (65%) evaluable patients had achieved bone marrow uMRD4 (5 positive and 2 not done) and 4 of 20 (20%) achieved bone marrow uMRD6. After 2 years, 2 additional patients achieved bone marrow uMRD4 (15 of 20 [75%]). Bone marrow flow and Clonoseq MRD data were available in 13 patients at the end of study. Eight were undetectable by flow and Clonoseq (uMRD6), 3 by flow only (uMRD4), and 2 were positive with both (MRD+).
Seven patients experienced 11 serious AEs attributed to treatment: 3 with sepsis, 3 pneumonia, 2 atrial fibrillation, and 1 each diarrhea, dehydration, and pulmonary embolism. AEs ≥ Grade 3 are shown in Table 2. There were no TLS events.
Conclusions: Ibr+ven combination for a fixed duration of 2 years after initial ibrutinib lead in is a well-tolerated, effective, oral, targeted therapy regimen for patients with rel/ref CLL. Most patients achieve an uMRD4 response in the bone marrow. The effect of poor risk features of disease and dose interruptions on depth of response will be reported.
Siddiqi: Janssen: Speakers Bureau; Oncternal: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Research Funding. Coutre: Acerta: Other: Data Safety Monitoring Committee, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitoring Committee, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal